

An Expression Makes a World of Difference CDK7 Portfolio Update October 17, 2019 Exhibit 99.2

Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including our ability to: advance the development of our programs, including SY-5609, under the timelines we project; demonstrate in any clinical trials the requisite safety, efficacy and combinability of SY-5609; successfully progress SY-5609 through IND-enabling preclinical and toxicology studies by year-end and initiate clinical development in the first quarter of 2020; replicate scientific and non-clinical data in clinical trials; successfully demonstrate that SY-5609 is a best-in-class CDK7 inhibitor; or that selective CDK7 inhibition can address difficult-to-treat cancers; obtain and maintain patent protection for SY-5609 and the freedom to operate under third party intellectual property; avail ourselves of accelerated regulatory pathways or obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties; manage competition; manage expenses; raise the substantial additional capital needed to achieve our business objectives; attract and retain qualified personnel; and successfully execute on our business strategies and long-term vision; risks described under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2018 and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2019, each of which is on file with the Securities and Exchange Commission (SEC); and risks described in other filings that we may make with the SEC in the future. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise.

Update on our selective CDK7 inhibitor portfolio Portfolio decision to prioritize SY-5609 and discontinue further development of SY-1365 based on a combination of factors, including: Superior preclinical profile of SY-5609, with best-in-class potential in emerging landscape of oral CDK7 inhibitors Initial clinical activity and tolerability data from the expansion that, despite showing some clinical activity, did not support an optimal profile of SY-1365 for patients As an oral molecule, we believe SY-5609 provides greater flexibility in dosing and greater opportunity to sustain CDK7 target coverage needed to improve treatment outcomes Based on these factors, we believe SY-5609 provides the best opportunity to realize the promise of selective CDK7 inhibition for patients
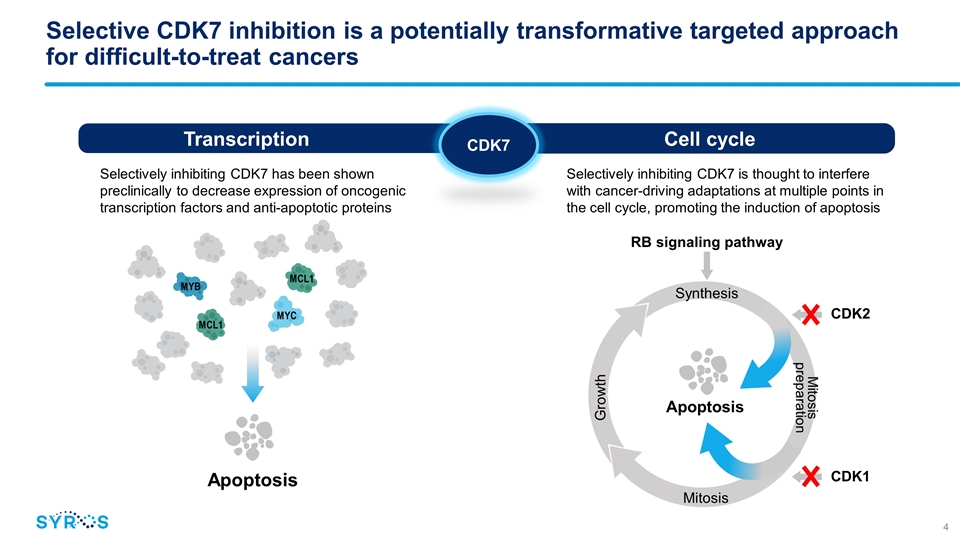
Selective CDK7 inhibition is a potentially transformative targeted approach for difficult-to-treat cancers Apoptosis Selectively inhibiting CDK7 has been shown preclinically to decrease expression of oncogenic transcription factors and anti-apoptotic proteins Selectively inhibiting CDK7 is thought to interfere with cancer-driving adaptations at multiple points in the cell cycle, promoting the induction of apoptosis MYC MYB MCL1 MCL1 Cell cycle Transcription CDK7 Apoptosis RB signaling pathway Synthesis Growth Mitosis preparation Mitosis CDK2 CDK1

Phase 1 clinical trial of SY-1365 Relapsed ovarian cancer, 3+ prior lines Single agent Relapsed ovarian cancer, 1+ prior lines (platinum sensitive) Combination with carboplatin Relapsed clear cell ovarian cancer Single agent Solid tumors accessible for biopsy Single agent HR+ metastatic breast cancer, CDK4/6 inhibitor resistant Combination with fulvestrant Open to all patients with advanced solid tumors Explored once and twice-a-week dosing Established proof-of-mechanism with early evidence of clinical activity, including a durable PR at dose of 80 mg/m2 and stable disease AEs were generally low-grade, including peri-infusional AEs, manageable and reversible Dose escalation Presented in November 2018 at EORTC-NCI-AACR Expansion Initiated at 53 mg/m2 in combination and 80 mg/m2 as single agent

Overview of single-agent safety data from dose escalation and expansion Majority of AEs were low-grade and reversible Most frequent AEs occurred on the day of infusion and are thought to be related to IV administration These peri-infusional AEs contributed to early discontinuations Lengthening infusion times reduced peak drug concentrations and appeared to reduce frequency and severity of peri-infusional AEs Data as of planned Sept. 30, 2019 data snapshot

SY-1365 overview of clinical response in expansion portion of Phase 1 trial Patients Treated N Response-Evaluable Population N Best Response N (%) Single Agent 53 31 (HGSOC 13; OCC 1; Biopsy 17) Stable Disease: 13 (42%) Combination 15 11 (Carboplatin 8; Fulvestrant 3) Stable Disease: 7 (64%) Majority of response-evaluable patients were treated at 53 and 64 mg/m2 Best response observed was stable disease Up to 214 days in the single agent setting Up to 337 days in the carboplatin combination Data as of planned Sept. 30, 2019 data snapshot

Apoptosis observed in tumor tissue in patients treated with SY-1365 Increases were observed in patients enrolled at doses of 53 mg/m2 and higher, with the highest increases observed in both patients enrolled at 80 mg/m2 One of the two patients enrolled at 80 mg/m2 is the previously reported durable PR from the dose escalation Average % CC3+ tumor cells [---32---] [----------53-----------] [---------64-----------] [----------80------------] Dosing Cohort (mg/m2) Data as of planned Sept. 30, 2019 data snapshot

SY-1365 summary Proof-of-mechanism established with early evidence of clinical activity Majority of AEs thought to be related to IV administration Based on preclinical and clinical data, we believe that: Sustained CDK7 target coverage is needed to enhance clinical activity Achieving sustained target coverage with an IV while managing tolerability would create an overly burdensome dosing schedule for patients These challenges can be better addressed with SY-5609

SY-5609 is a superior development candidate, with best-in-class potential SY-1365 SY-5609 First-in-class ü Convenience ü Potency ü Selectivity ü Dosing flexibility ü Preclinical anti-tumor activity ü

SY-5609: A highly selective and potent non-covalent oral CDK7 inhibitor Only 4 of 485 kinases inhibited at ≥90% at 1uM 13,000- to 49,000-fold more selective for CDK7 over CDK2, CDK9 and CDK12 SY-5609 SY-1365 CDK7 potency Kd = 0.059 nM KI = 20 nM CDK12 (fold) CDK2 (fold) CDK9 (fold) 13,000x 49,000x 16,000x 5x 53x 23x SY-5609 is more selective and potent than SY-1365 100-91% Inhibition 90-80% Inhibition 79-71% Inhibition Data presented in April 2019 at AACR Annual Meeting

SY-5609 shows tumor growth inhibition, including regressions, below MTD Regressions observed at 5-fold below MTD of ≥10 mg/kg QD Triple negative breast cancer model Dashed lines represent end of treatment Days Post Treatment Initiation

SY-5609 shows robust anti-tumor activity, including complete regressions, in multiple PDX models Triple-negative breast cancer model High-grade serous ovarian cancer model Small-cell lung cancer model Dashed lines represent end of treatment Vehicle 10mpk QD 5mpk BID Days Post Treatment Initiation Vehicle 3mpk BID Vehicle 3mpk BID

SY-5609 shows greater anti-tumor activity than SY-5609 in head-to-head preclinical study Pancreatic cancer PDX model SY-5609 induces 100% tumor growth inhibition at doses below MTD By comparison, SY-1365 induces modest tumor growth inhibition at MTD Vehicle SY-1365 40 mg/kg BIW, IV SY-5609 6 mg/kg QD, PO

Expect to initiate Phase 1 trial in select solid tumors in Q1 2020 On track to complete IND-enabling studies by year-end Dose escalation will enroll patients with select solid tumors Focusing on patient populations we believe are enriched for response, including: Breast Lung Ovarian Solid tumors of any histology defined by a specific molecular signature

Key takeaways Selective CDK7 inhibition is a potentially transformative targeted approach for difficult-to-treat cancers SY-1365 demonstrated early evidence of clinical activity and we believe more sustained CDK7 target coverage is needed to improve treatment outcomes SY-5609 is a superior development candidate with best-in-class potential Oral allows dosing flexibility with potential for sustained target coverage and improved tolerability Prioritizing SY-5609 allows us to focus where we believe we have the greatest opportunity to deliver on the promise of CDK7 inhibition for patients We expect to initiate a Phase 1 trial in Q1 2020 in patients with select solid tumors, including breast, lung and ovarian cancers and cancers with a specific molecular signature
