

Key Opinion Leader Symposium on Selective CDK7 Inhibition in Ovarian and Breast Cancers April 24, 2019 Anne Ovarian cancer survivor Exhibit 99.1

Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including whether or when Incyte will exercise any of its options or any option exercise fees, milestone payments or royalties under the Incyte collaboration will ever be paid, our ability to: advance the development of our programs, including SY-1425 and SY-1365, under the timelines we project in current and future clinical trials or to achieve clinical proof of concept in these trials; demonstrate in any current and future clinical trials the requisite safety, efficacy and combinability of our drug candidates; successfully progress SY-5609 through IND-enabling preclinical and toxicology studies; replicate scientific and non-clinical data in clinical trials; successfully develop a companion diagnostic test to identify patients with the RARA and IRF8 biomarkers; obtain and maintain patent protection for our drug candidates and the freedom to operate under third party intellectual property; avail ourselves of accelerated regulatory pathways or obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties, including our ability to perform under the collaboration agreement with Incyte; manage competition; manage expenses; raise the substantial additional capital needed to achieve our business objectives; attract and retain qualified personnel; and successfully execute on our business strategies and long-term vision; risks described under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2018 that is on file with the Securities and Exchange Commission (SEC); and risks described in other filings that we may make with the SEC in the future. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise.

Company Overview Nancy Simonian, M.D.

Syros today: Clear vision, growing pipeline, pioneering platform Leading gene control platform Two first-in-class clinical-stage programs Clinical trials in six cancer patient populations Three fast-to-market strategies Multiple data readouts in 2019 and 2020 Experienced leadership team

98% Previously unexplored regulatory regions of the genome control the expression of genes determining cell function; majority of disease variation found in these regions Patient Impact Medicines that control the expression of genes to provide a profound benefit for patients with severe diseases Pioneering a new approach: Medicines that control the expression of genes Our leading gene control platform Regulatory Genomics Disease Biology Transcriptional Chemistry !

Deep and growing pipeline with multiple potential first-in-class programs SY-1425 is approved in Japan as Amnolake® (tamibarotene) for patients with relapsed/refractory APL * Expected to begin in Q3 2019 Program Indication Drug Discovery IND- Enabling Early Clinical Mid- Clinical Pivotal Commercial Rights RARα agonism SY-1425 (RARα agonist) Newly diagnosed unfit AML Syros (North America and Europe) Relapsed or refractory AML* CDK Inhibition SY-1365 (CDK7 inhibitor) High-grade serous ovarian cancer Syros (Global) Ovarian clear cell cancer HR+ breast cancer SY-5609 (Oral CDK7 inhibitor) Cancer CDK12/13 Inhibitor Cancer Cancer Macrophage target Immuno-oncology Cancer/Immuno-oncology Monogenic Disease Undisclosed target Sickle cell disease Monogenic diseases MPNs Myeloproliferative neoplasms Incyte (Global)

Realizing our vision for the promise of selective CDK7 inhibition in difficult-to-treat cancers Now SY-1365 in multiple ovarian and breast cancer patient populations Opportunities for rapid proof-of concept in high grade serous and clear cell ovarian cancers Next Combination data in earlier lines of therapy Additional solid tumors Blood cancers SY-5609 Vision Transformative targeted approach for a range of difficult-to-treat solid tumors and blood cancers

SY-1425 SY-1365 SY-5609 Three potential proof-of-concept data readouts in 2020 Multiple clinical milestones expected in 2019 and 2020 Q2 2019 Q3 2019 Q4 2019 2020 Complete enrollment in aza combo cohort in biomarker-positive ND unfit AML patients Open new aza combo cohort in R/R biomarker-positive AML Updated aza combo data in ND unfit AML Potential POC data on aza combo in R/R biomarker-positive AML Open new cohort in relapsed ovarian clear cell cancer Initial expansion data initial safety & efficacy from 3+ prior lines cohort Initial safety & PK on carbo combo initial safety, efficacy & mechanistic data from biopsy cohort Potential POC data in clear cell and in 3+ prior lines Additional data from carbo combo cohort and biopsy cohort; initial data from HR+ breast cancer cohort Complete IND-enabling studies Initiate Phase 1 oncology trial in early 2020

Overview of Syros’ CDK7 Franchise David Roth, M.D.

Selective CDK7 inhibition attacks two fundamental processes in cancer Apoptosis Selectively inhibiting CDK7 has been shown preclinically to decrease expression of oncogenic transcription factors and anti-apoptotic proteins Selectively inhibiting CDK7 is thought to interfere with cancer-driving adaptations at multiple points in the cell cycle, promoting the induction of apoptosis MYC MYB MCL1 MCL1 Cell cycle Transcription CDK7 Apoptosis RB signaling pathway Synthesis Growth Mitosis preparation Mitosis CDK2 CDK1

Exploring SY-1365 as single agent and in combination in ovarian and breast cancer patients, including two potential fast-to-market strategies Initial data from relapsed HGSOC and biopsy cohorts expected in Q4 2019, with additional data expected in 2020 Potential proof-of-concept data in clear cell and relapsed HGSOC (3+ prior lines) expected in 2020 Initial data from HR-positive breast cancer cohort expected in 2020 Ongoing expansion cohorts Relapsed HGSOC, 3+ prior lines Single agent N=24 Relapsed HGSOC, 1+ prior lines (platinum sensitive) Combination with carboplatin N=24 Relapsed ovarian clear cell cancer Single agent N=12 Solid tumors accessible for biopsy Single agent N=30 HR+ metastatic breast cancer, CDK4/6 inhibitor resistant Combination with fulvestrant N=12 Target enrollment Single/combo agent Patient population

SY-1365 demonstrated proof-of-mechanism at tolerable doses in dose escalation portion of ongoing Phase 1 trial Relative Expression (Log2) 3h post-dose vs. pre-dose SY-1365 Dose (mg/m2) 0 -1 -2 -3 1 4 8 16 32 53 64 80 107 PBMC Gene Expression Signature PBMC CDK7 Occupancy Exceeded desired target occupancy levels at doses ≥ 32 mg/m2 Most frequent related AEs include headache, nausea, vomiting and fatigue No reports of neutropenia Demonstrated dose-dependent downstream changes in gene expression Adverse events (AEs) were predominantly low grade, reversible and generally manageable Data as of October 15, 2018; Presented in November 2018 at EORTC-NCI-AACR Symposium
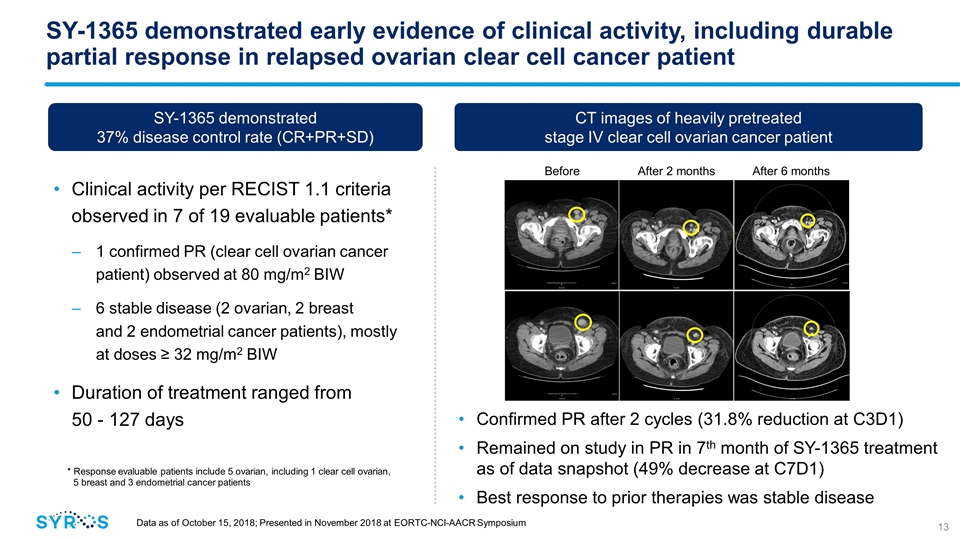
SY-1365 demonstrated early evidence of clinical activity, including durable partial response in relapsed ovarian clear cell cancer patient Clinical activity per RECIST 1.1 criteria observed in 7 of 19 evaluable patients* 1 confirmed PR (clear cell ovarian cancer patient) observed at 80 mg/m2 BIW 6 stable disease (2 ovarian, 2 breast and 2 endometrial cancer patients), mostly at doses ≥ 32 mg/m2 BIW Duration of treatment ranged from 50 - 127 days Data as of October 15, 2018; Presented in November 2018 at EORTC-NCI-AACR Symposium Before treatment After 2 months After 6 months CT images of heavily pretreated stage IV clear cell ovarian cancer patient SY-1365 demonstrated 37% disease control rate (CR+PR+SD) Confirmed PR after 2 cycles (31.8% reduction at C3D1) Remained on study in PR in 7th month of SY-1365 treatment as of data snapshot (49% decrease at C7D1) Best response to prior therapies was stable disease * Response evaluable patients include 5 ovarian, including 1 clear cell ovarian, 5 breast and 3 endometrial cancer patients

RB pathway alterations predict response to SY-1365 in ovarian cancer PDX models, pointing to potential biomarker-driven patient enrichment strategy Approximately 2/3 of high-grade serous ovarian cancer patients have RB alterations1 Approximately half of ovarian clear cell cancer patients have deleted or amplified genes in RB or cell cycle pathways2 Approximately 1/3 of HR+ breast cancer patients acquire RB or cell cycle alterations post CDK4/6 inhibitors3 1TCGA Ovarian Cancer Integrated Analysis, Nature 2011 2 Murakami et al., Am J Pathol. 2017 Oct;187(10):2246-2258 3 Spring et al., San Antonio Breast Cancer Symposium 2018 Ovarian Subtype Response Class % TGI Clear Cell Responder 86 Serous Responder 84 Serous Responder 81 Serous Responder 75 Serous Responder 74 Serous Responder 74 Serous Responder 62 Serous Responder 55 Serous Responder 51 Serous Non Responder 34 90% of models with RB alterations responded to SY-1365 Data presented in April 2019 at AACR Annual Meeting RB pathway alterations prospectively defined per TCGA criteria – including RB1 deletion or mutation, CDKN2A downregulation or deletion, CCNE1 amplification, CCND1 amplification, or CCND2 upregulation Supports ongoing development in ovarian and CDK4/6 inhibitor-resistant breast cancer patients

SY-1365 shows anti-tumor activity as single agent and in combination with standard-of-care in ovarian models, supporting ongoing clinical investigation Weekly SY-1365 in combination with carboplatin enhances activity in ovarian cancer xenograft models SY-1365 induces tumor growth inhibition, including complete regressions, in heavily pretreated ovarian cancer models Tumor Volume (mm3) Days Historical/untreated Treated 40mg/kg BIW 30mg/kg BIW Data presented in April 2018 at the American Association of Cancer Research (AACR) Annual Meeting Data presented in November 2018 at EORTC-NCI-AACR Symposium Vehicle QW*3 30mpk SY-1365 QW 50mpk Carboplatin QW 50mpk Carboplatin + 30mpk SY-1365 QW 0 10 20 0 300 600 900 1200 1500 Days Dosing Tumor Volume (mm3) SY-1365 inhibited DNA repair and transcription of HRR genes in preclinical models, inducing an HRD-like state that may increase sensitivity to DNA-damaging agents and DNA repair inhibitors Responses observed in 10/17 (59%) models, irrespective of BRCA status or PARP inhibitor sensitivity

SY-1365 shows anti-tumor activity and synergy with fulvestrant in HR-positive breast cancer models, including CDK4/6 inhibitor resistant models Data presented by Syros’ collaborators at Dana-Farber Cancer Institute in December 2018 at San Antonio Breast Cancer Symposium SY-1365 inhibits growth of CDK4/6 inhibitor resistant breast cancer cell models Growth Fold Change 25 20 15 10 5 0 0 3 6 9 Days DMSO Palbociclib 1uM SY-1365 1nM SY-1365 10nM Palbociclib 1uM+SY-1365 1nM Palbociclib 1uM+SY-1365 10nM In vitro synergy was seen in several HR+ breast cancer cell lines HR-positive CDX model 600 500 400 300 200 100 0 0 5 10 15 20 25 Tumor volume (mm3) Days Vehicle Fulvestrant 5mg/kg QW SY-1365 30mg/kg BIW Fulvestrant + SY-1365 Source: Jeselsohn et al, 2018; Data from collaboration with Dana-Farber Cancer Institute

SY-5609: A potent and highly selective oral CDK7 inhibitor SY-5609 is a potent and highly selective oral CDK7 inhibitor SY-5609 induced apoptosis in cancer cells but not in non-cancerous cells Significant opportunity for adding an oral approach across a range of solid tumors and blood cancers Expect to initiate a Phase 1 oncology trial for SY-5609 in early 2020 Only 4 of 485 kinases inhibited at > 90% 13,000- to 49,000-fold greater selectivity for CDK7 over other CDK family members 100-91% Inhibition 90-80% Inhibition 79-71% Inhibition Data presented in April 2019 at AACR Annual Meeting DMSO SY-5609 100nM SY-5609 250nM SY-5609 500nM Non-cancerous cells TNBC cells %Annexin V/PI + 60 50 40 30 20 10 0

200 SY-5609 demonstrates substantial tumor growth inhibition as a single agent in multiple breast and ovarian cancer models Triple Negative Breast Cancer CDX Model Ovarian Cancer CDX Model Complete regressions observed in multiple TNBC and ovarian CDX models at doses below the MTD Modulation of downstream markers, including MCL1, observed in tumor tissues, confirming CDK7 inhibition in vivo Substantial tumor growth inhibition also observed in multiple PDX models with doses below MTD Data presented in April 2019 at AACR Annual Meeting Days 1800 1600 1400 1200 1000 800 600 400 0 0 4 8 12 16 20 24 28 32 36 Tumor Volume (mm3) Vehicle, p.o., BID SY-5609 2.5 mg/kg p.o. BID SY-5609 5 mg/kg p.o. BID 0 5 10 15 20 25 30 35 1600 1400 1200 1000 800 600 400 200 0 Days Tumor Volume (mm3) Vehicle, p.o., BID SY-5609 2.5 mg/kg p.o. BID SY-5609 5 mg/kg p.o. BID Last dose Last dose

Key takeaways Selective CDK7 inhibition represents a potentially transformative targeted approach for many difficult-to-treat cancers SY-1365 is a first-in-class selective CDK7 inhibitor in Phase 1 trial Initial development is focused on ovarian and breast cancers based on mechanistic rationale, preclinical data and unmet need and supported by early clinical signals Unmet need in relapsed ovarian and metastatic HR-positive breast cancers is significant Ongoing Phase 1 trial supports potential fast-to-market strategies and opportunities to move into earlier lines of treatment and emerging patient populations Syros is building on its CDK7 leadership with SY-5609, a highly selective oral CDK7 inhibitor, that together with SY-1365 could make for a powerful CDK7 franchise
