Exhibit 99.2
Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including whether or when Incyte will exercise any of its options or any option exercise fees, milestone payments or royalties under the Incyte collaboration will ever be paid, and Syros’ ability to: advance the development of its programs, including SY-1425 and SY-1365, under the timelines it projects in current and future clinical trials; advance discovery programs to identify drug candidates for IND-enabling studies; obtain and maintain patent protection for its drug candidates and the freedom to operate under third party intellectual property; demonstrate in any current and future clinical trials the requisite safety, efficacy and combinability of its drug candidates; replicate scientific and non-clinical data in clinical trials; successfully develop a companion diagnostic test to identify patients with RARA and IRF8 biomarkers; obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties; manage competition; manage expenses; raise the substantial additional capital needed to achieve its business objectives; attract and retain qualified personnel; and successfully execute on its business strategies; risks described under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2017 that is on file with the Securities and Exchange Commission (SEC); and risks described in other filings that we may make with the SEC in the future. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise.

To create unparalleled value for patients, employees and shareholders by creating transformative medicines for severe disease through our world-leading expertise in gene control and our exceptional people and culture Our Vision

Syros Productive Advancing 1 IND every other year on average Pioneering First platform dedicated to the regulatory genome Strong foundation Broad strategic optionality driven by experienced leadership team Broad impact Platform applicable across a wide array of diseases with focus on cancer, I/O and monogenic diseases 2 clinical-stage programs and robust preclinical pipeline in 4 years Rapid translation
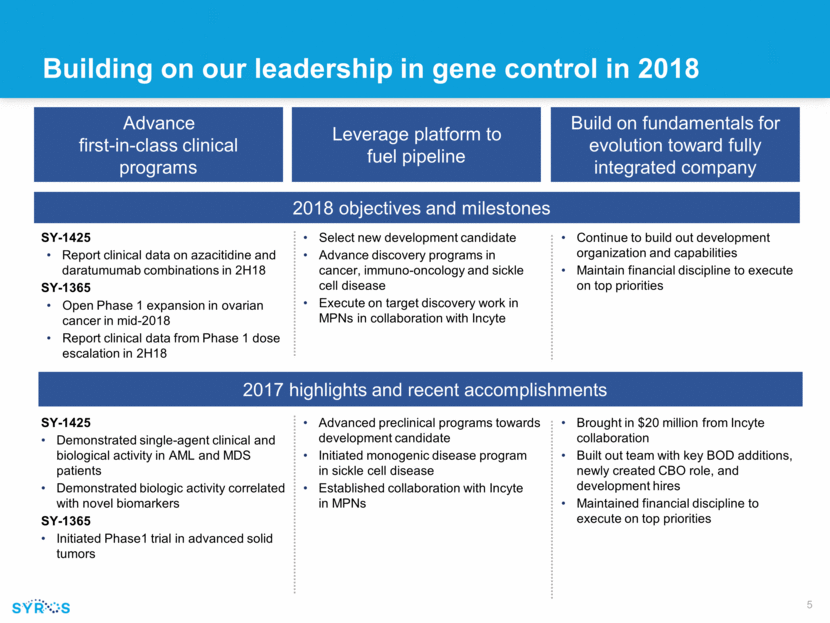
Building on our leadership in gene control in 2018 Advance first-in-class clinical programs Leverage platform to fuel pipeline Build on fundamentals for evolution toward fully integrated company 2018 objectives and milestones SY-1425 Report clinical data on azacitidine and daratumumab combinations in 2H18 SY-1365 Open Phase 1 expansion in ovarian cancer in mid-2018 Report clinical data from Phase 1 dose escalation in 2H18 Continue to build out development organization and capabilities Maintain financial discipline to execute on top priorities Select new development candidate Advance discovery programs in cancer, immuno-oncology and sickle cell disease Execute on target discovery work in MPNs in collaboration with Incyte 2017 highlights and recent accomplishments SY-1425 Demonstrated single-agent clinical and biological activity in AML and MDS patients Demonstrated biologic activity correlated with novel biomarkers SY-1365 Initiated Phase1 trial in advanced solid tumors Brought in $20 million from Incyte collaboration Built out team with key BOD additions, newly created CBO role, and development hires Maintained financial discipline to execute on top priorities Advanced preclinical programs towards development candidate Initiated monogenic disease program in sickle cell disease Established collaboration with Incyte in MPNs
Gene Control Platform

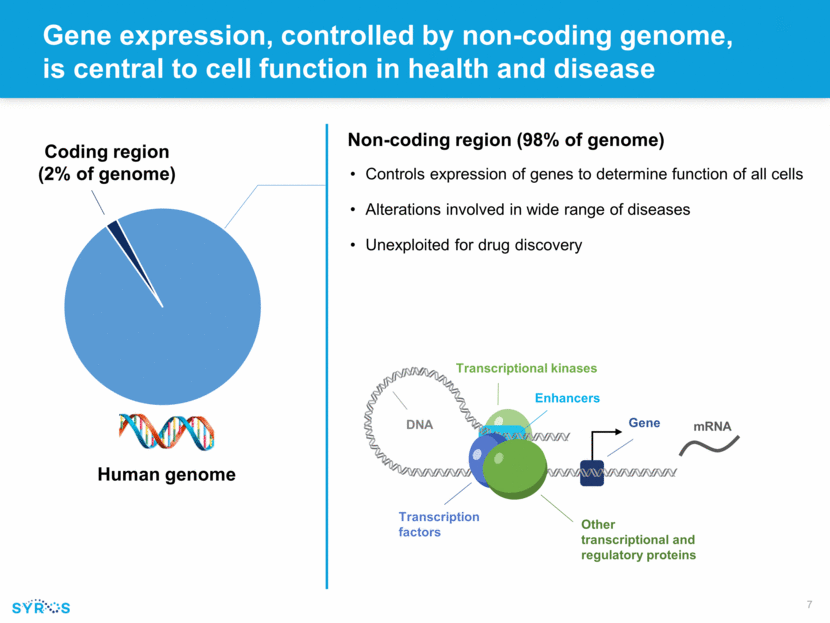
Gene expression, controlled by non-coding genome, is central to cell function in health and disease Human genome Coding region (2% of genome) Non-coding region (98% of genome) Controls expression of genes to determine function of all cells Alterations involved in wide range of diseases Unexploited for drug discovery Enhancers Transcription factors Other transcriptional and regulatory proteins Transcriptional kinases Gene DNA mRNA
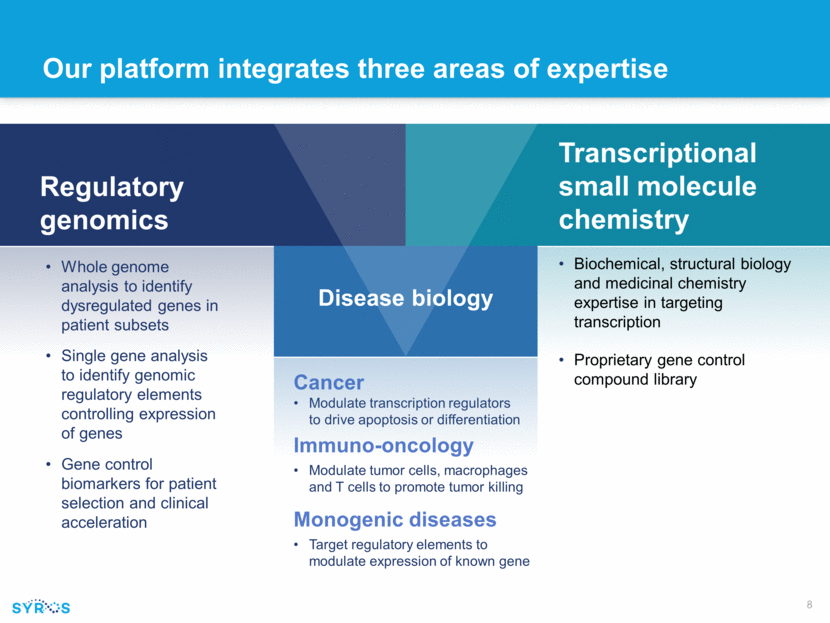
Our platform integrates three areas of expertise Cancer Modulate transcription regulators to drive apoptosis or differentiation Immuno-oncology Modulate tumor cells, macrophages and T cells to promote tumor killing Monogenic diseases Target regulatory elements to modulate expression of known gene Regulatory genomics Whole genome analysis to identify dysregulated genes in patient subsets Single gene analysis to identify genomic regulatory elements controlling expression of genes Gene control biomarkers for patient selection and clinical acceleration Transcriptional small molecule chemistry Biochemical, structural biology and medicinal chemistry expertise in targeting transcription Proprietary gene control compound library Disease biology
Our Programs

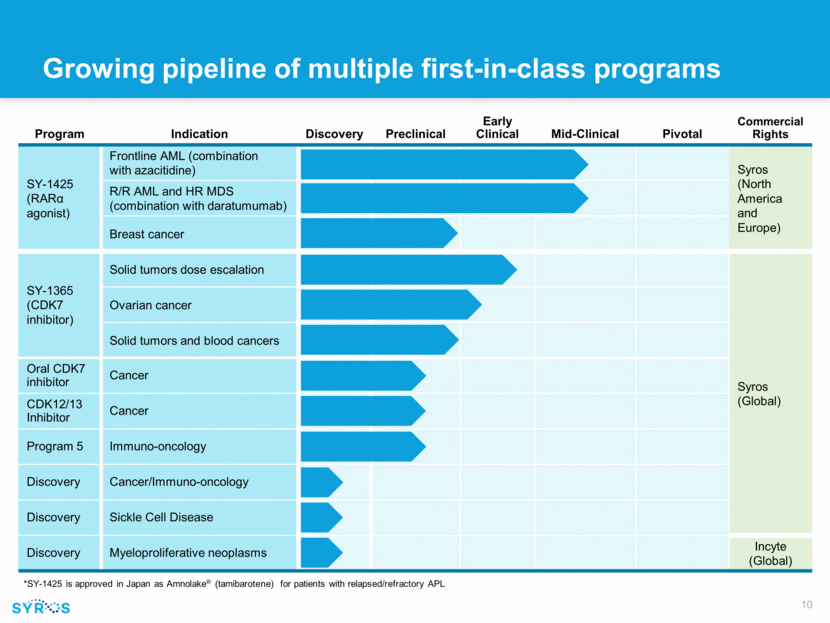
Program Indication Discovery Preclinical Early Clinical Mid-Clinical Pivotal Commercial Rights SY-1425 (RAR agonist) Frontline AML (combination with azacitidine) Syros (North America and Europe) R/R AML and HR MDS (combination with daratumumab) Breast cancer SY-1365 (CDK7 inhibitor) Solid tumors dose escalation Syros (Global) Ovarian cancer Solid tumors and blood cancers Oral CDK7 inhibitor Cancer CDK12/13 Inhibitor Cancer Program 5 Immuno-oncology Discovery Cancer/Immuno-oncology Discovery Sickle Cell Disease Discovery Myeloproliferative neoplasms Incyte (Global) *SY-1425 is approved in Japan as Amnolake® (tamibarotene) for patients with relapsed/refractory APL Growing pipeline of multiple first-in-class programs
SY-1425 (RAR agonist): Turning on differentiation genes in cancer First-in-class selective, oral RAR agonist Novel AML and MDS subsets and biomarkers discovered by Syros In Phase 2 trial in combination with azacitidine and daratumumub Data expected 2H 2018 Single-agent activity and myeloid differentiation in biomarker-positive AML and MDS patients support ongoing development in combination Chronic, daily dosing generally well-tolerated Significant market potential AML and MDS continue to be areas of high unmet need Few options for newly diagnosed, unfit AML and R/R AML and HR MDS Potential in additional AML and HR MDS subsets and other RARA-positive cancers, including breast Acute Myelogenous Leukemia (AML) and Myelodysplastic Syndromes (MDS)
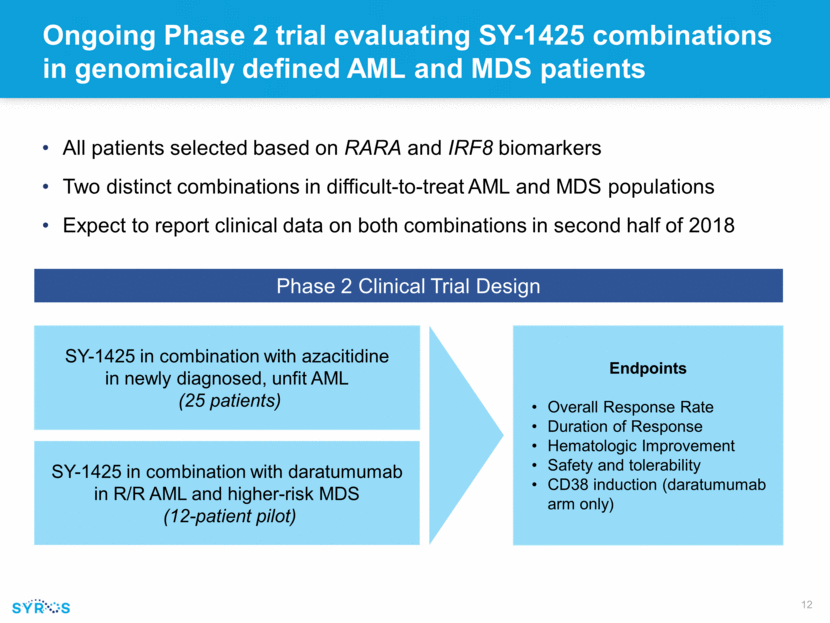
Ongoing Phase 2 trial evaluating SY-1425 combinations in genomically defined AML and MDS patients All patients selected based on RARA and IRF8 biomarkers Two distinct combinations in difficult-to-treat AML and MDS populations Expect to report clinical data on both combinations in second half of 2018 SY-1425 in combination with azacitidine in newly diagnosed, unfit AML (25 patients) SY-1425 in combination with daratumumab in R/R AML and higher-risk MDS (12-patient pilot) Endpoints Overall Response Rate Duration of Response Hematologic Improvement Safety and tolerability CD38 induction (daratumumab arm only) Phase 2 Clinical Trial Design
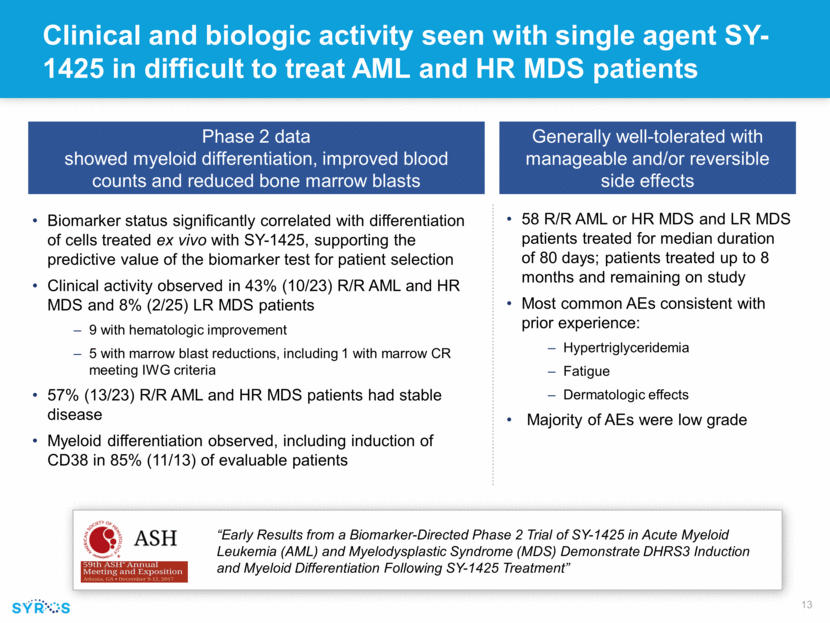
Clinical and biologic activity seen with single agent SY-1425 in difficult to treat AML and HR MDS patients Generally well-tolerated with manageable and/or reversible side effects 58 R/R AML or HR MDS and LR MDS patients treated for median duration of 80 days; patients treated up to 8 months and remaining on study Most common AEs consistent with prior experience: Hypertriglyceridemia Fatigue Dermatologic effects Majority of AEs were low grade Phase 2 data showed myeloid differentiation, improved blood counts and reduced bone marrow blasts Biomarker status significantly correlated with differentiation of cells treated ex vivo with SY-1425, supporting the predictive value of the biomarker test for patient selection Clinical activity observed in 43% (10/23) R/R AML and HR MDS and 8% (2/25) LR MDS patients 9 with hematologic improvement 5 with marrow blast reductions, including 1 with marrow CR meeting IWG criteria 57% (13/23) R/R AML and HR MDS patients had stable disease Myeloid differentiation observed, including induction of CD38 in 85% (11/13) of evaluable patients “Early Results from a Biomarker-Directed Phase 2 Trial of SY-1425 in Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) Demonstrate DHRS3 Induction and Myeloid Differentiation Following SY-1425 Treatment”
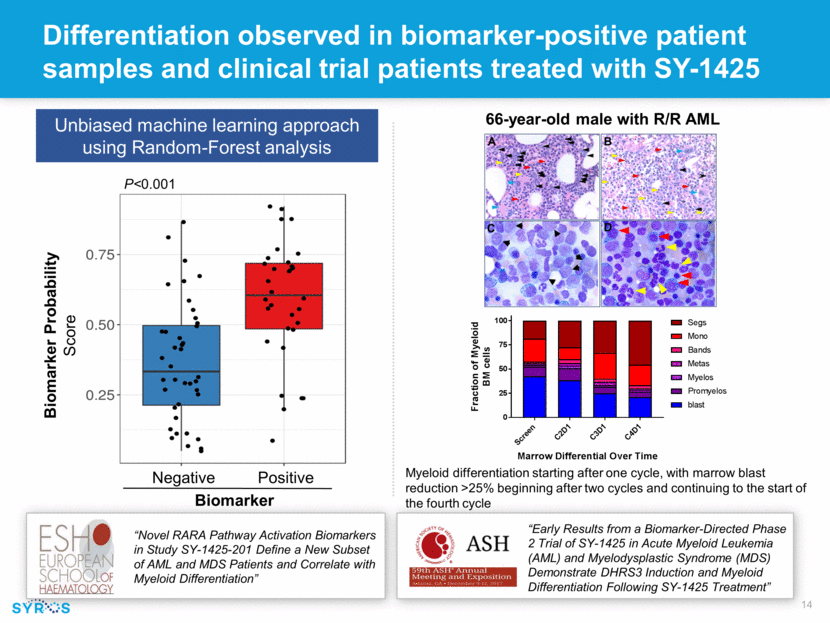
Differentiation observed in biomarker-positive patient samples and clinical trial patients treated with SY-1425 Myeloid differentiation starting after one cycle, with marrow blast reduction >25% beginning after two cycles and continuing to the start of the fourth cycle 66-year-old male with R/R AML Unbiased machine learning approach using Random-Forest analysis Negative Positive Biomarker “Early Results from a Biomarker-Directed Phase 2 Trial of SY-1425 in Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) Demonstrate DHRS3 Induction and Myeloid Differentiation Following SY-1425 Treatment” Biomarker Probability Score P<0.001 “Novel RARA Pathway Activation Biomarkers in Study SY-1425-201 Define a New Subset of AML and MDS Patients and Correlate with Myeloid Differentiation”
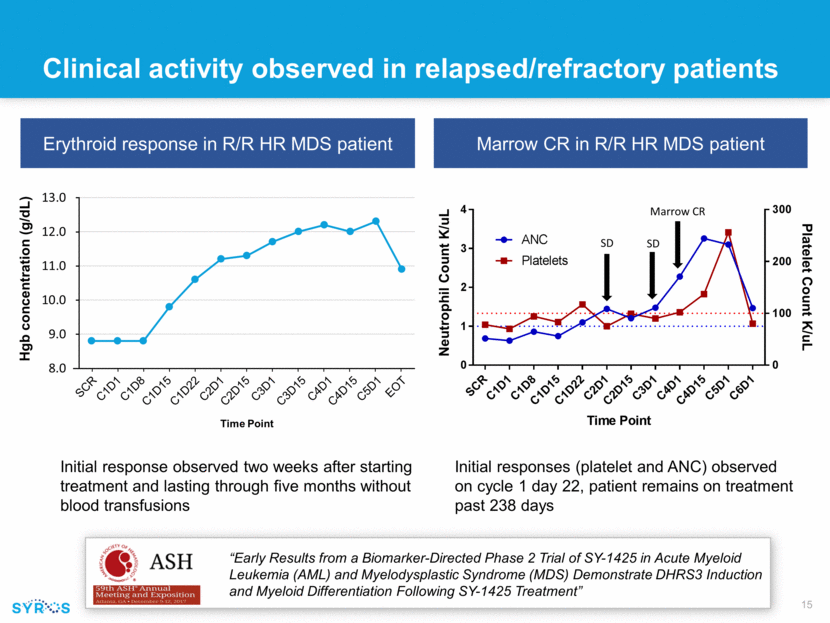
Clinical activity observed in relapsed/refractory patients Initial response observed two weeks after starting treatment and lasting through five months without blood transfusions Erythroid response in R/R HR MDS patient Marrow CR in R/R HR MDS patient Initial responses (platelet and ANC) observed on cycle 1 day 22, patient remains on treatment past 238 days “Early Results from a Biomarker-Directed Phase 2 Trial of SY-1425 in Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) Demonstrate DHRS3 Induction and Myeloid Differentiation Following SY-1425 Treatment” Marrow CR SD SD Neutrophil Count K/uL Platelet Count K/uL SCR C1D1 C1D8 C1D15 C1D22 C2D1 C2D15 C3D1 C3D15 C4D1 C4D15 C5D1 EOT 8.0 9.0 10.0 11.0 12.0 13.0 SCR C1D1 C1D8 C1D15 C1D22 C2D1 C2D15 C3D1 C3D15 C4D1 C4D15 C5D1 EOT Hgb concentration (g/ dL ) Time Point S C R C 1 D 1 C 1 D 8 C 1 D 1 5 C 1 D 2 2 C 2 D 1 C 2 D 1 5 C 3 D 1 C 4 D 1 C 4 D 1 5 C 5 D 1 C 6 D 1 0 1 2 3 4 0 100 200 300 Time Point N e u t r o p h i l C o u n t K / m L P l a t e l e t C o u n t K / m L ANC Platelets
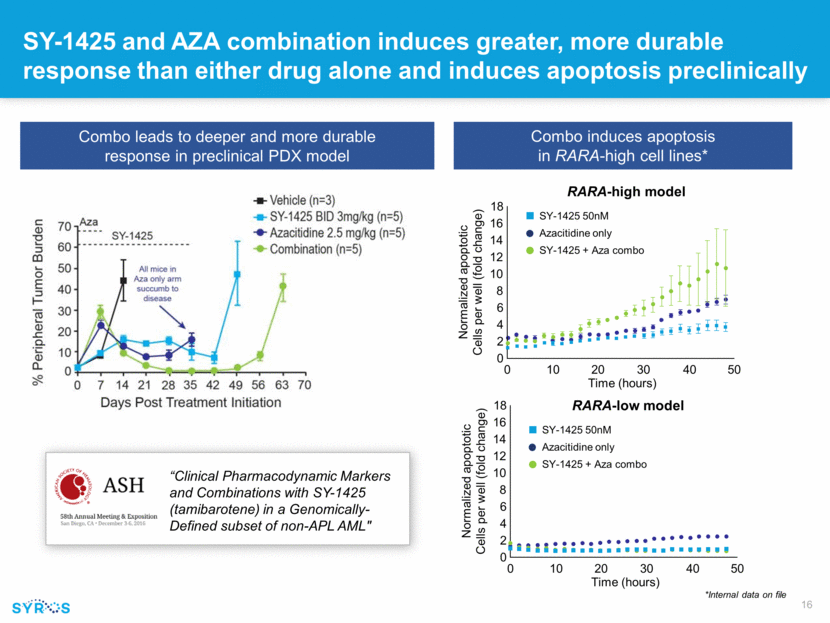
SY-1425 and AZA combination induces greater, more durable response than either drug alone and induces apoptosis preclinically *Internal data on file Combo leads to deeper and more durable response in preclinical PDX model Combo induces apoptosis in RARA-high cell lines* “Clinical Pharmacodynamic Markers and Combinations with SY-1425 (tamibarotene) in a Genomically-Defined subset of non-APL AML" RARA-high model RARA-low model 18 16 14 12 10 8 6 4 2 0 0 10 20 30 40 50 Time (hours) Normalized apoptotic Cells per well (fold change) 18 16 14 12 10 8 6 4 2 0 0 10 20 30 40 50 Time (hours) Normalized apoptotic Cells per well (fold change) SY-1425 50nM Azacitidine only SY-1425 + Aza combo SY-1425 50nM Azacitidine only SY-1425 + Aza combo
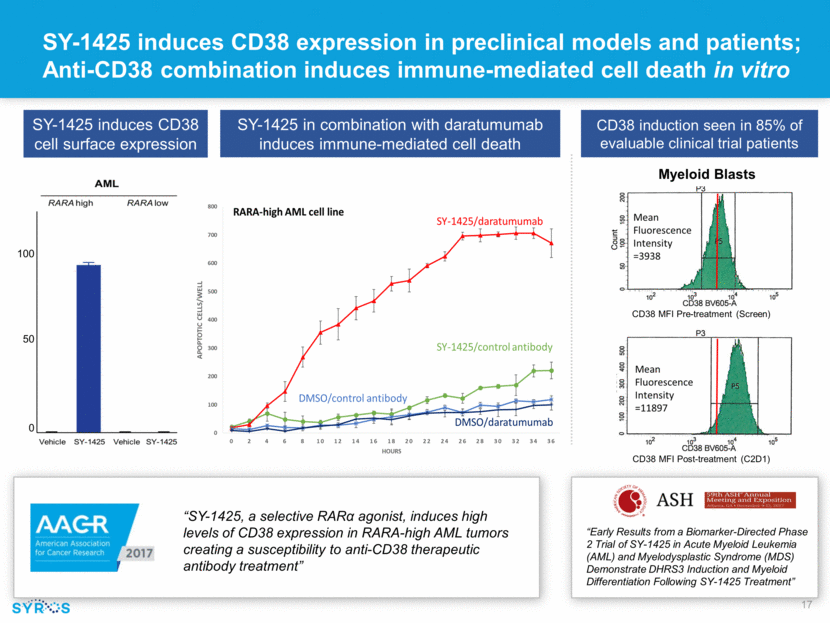
SY-1425 in combination with daratumumab induces immune-mediated cell death “SY-1425, a selective RAR agonist, induces high levels of CD38 expression in RARA-high AML tumors creating a susceptibility to anti-CD38 therapeutic antibody treatment” SY-1425 induces CD38 cell surface expression SY-1425 induces CD38 expression in preclinical models and patients; Anti-CD38 combination induces immune-mediated cell death in vitro 100 50 0 CD38 induction seen in 85% of evaluable clinical trial patients Myeloid Blasts CD38 MFI Pre-treatment (Screen) CD38 MFI Post-treatment (C2D1) “Early Results from a Biomarker-Directed Phase 2 Trial of SY-1425 in Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) Demonstrate DHRS3 Induction and Myeloid Differentiation Following SY-1425 Treatment”
Significant need for well-tolerated oral therapies that extend survival and improve quality of life Newly diagnosed, unfit >50% of AML patients ineligible for standard chemo upon diagnosis HMAs/ less intensive therapies with modest efficacy are standard-of-care Survival of < 12 months Relapsed or refractory Newly approved agents target limited subsets of patients with modest efficacy Patients progress quickly in relapsed setting Survival of < 6 months Newly diagnosed higher-risk Most patients treated with HMAs/ less intensive therapies that have modest efficacy Survival of 0.8-1.6 years Relapsed or refractory higher-risk Few treatment options with limited efficacy No new drugs approved since 2006 Survival of < 6 months AML incidence1: ~33,000 HR MDS incidence1: ~7,500 1 Incidence figures include annual diagnoses in the U.S., Canada and the EU 5 (UK, Germany, France, Spain and Italy). Health Advances analysis. Sources: Expert Rev. Pharmacoecon. Outcomes Res. Early online, 1–10 (2015); Clinical Lymphoma, Myeloma & Leukemia, Vol. 16, No. 11, 625-36; Blood 2012 120:2454-2465. NCCN Guidelines 2017 MDS. ~ 33% RARA or IRF8 biomarker positive ~ 33% RARA or IRF8 biomarker positive
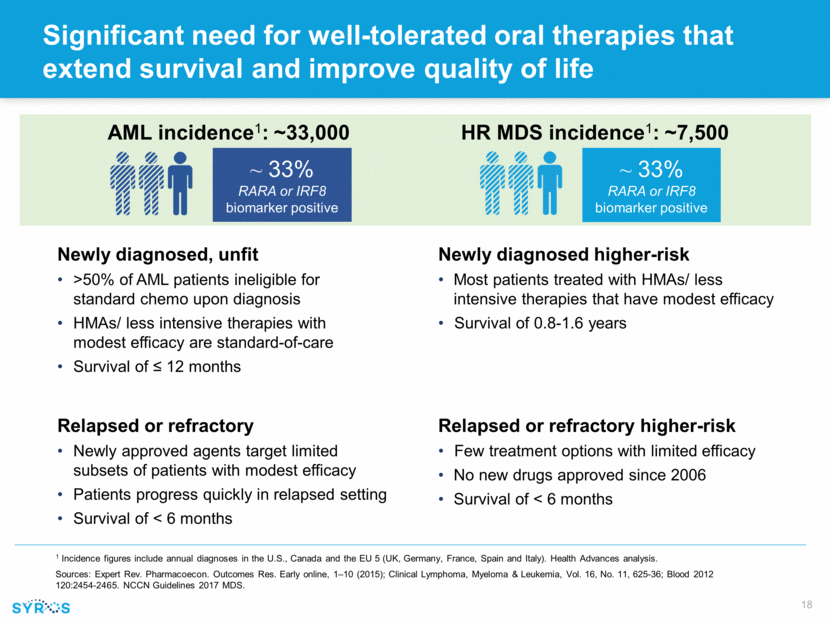
Unique mechanism and tolerability profile support combination potential RARA and IRF8 biomarkers and SY-1425 opportunity cut across mutational landscape Unique mechanism points to potential for combinations with chemo and targeted agents without anticipated overlapping toxicities ~20% FLT3+ ~33% RARA/IRF8 biomarker ~15% IDH+ Total AML Estimated frequency of RARA/IRF8 biomarkers based on Syros’ analysis of screed patients in ongoing trial, and estimated frequency of IDH and FLT3 mutations based on published studies
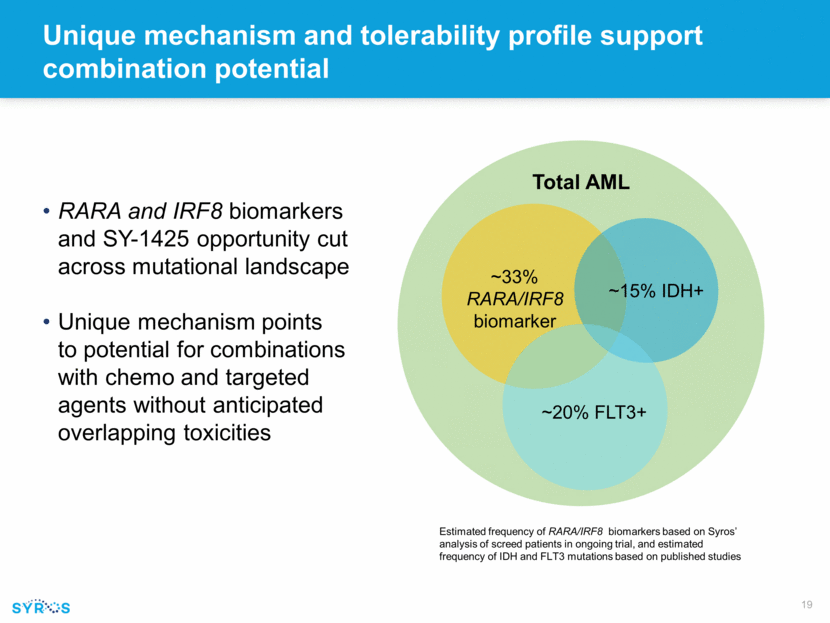
Current and future opportunities for SY-1425 within AML and HR MDS treatment landscape Source: Health Advances interviews and analysis, SEER 2015, UpToDate 2015, Fernandez HF 2010 ASH, Ohtake S 2004 Blood. SY-1425 current targeted segments AML Patients Unfit AML R/R AML HR MDS Patients Fit AML HR MDS 7+3 or Vyxeos +/- Rydapt +/- Mylotarg HMAs Low-dose chemo Clinical trial R/R HR MDS SY-1425 future potential segments HMAs Supportive Care Clinical trial Idhifa Mylotarg HMAs Supportive Care Clinical trial
SY-1365 (CDK7 inhibitor): Controlling expression of tumor-driving genes First-in-class selective inhibitor of CDK7 Lowers expression of key tumor-driving genes, transcription factors and anti-apoptotic proteins CDK7 inhibition induces apoptosis and preferentially kills cancer cells over non-cancerous cells Currently in Phase 1 clinical trial with planned expansion into ovarian cancer Data from dose escalation phase expected in second half of 2018 Broad potential to expand into additional solid tumors and blood cancers Difficult-to-treat solid tumors and blood cancers
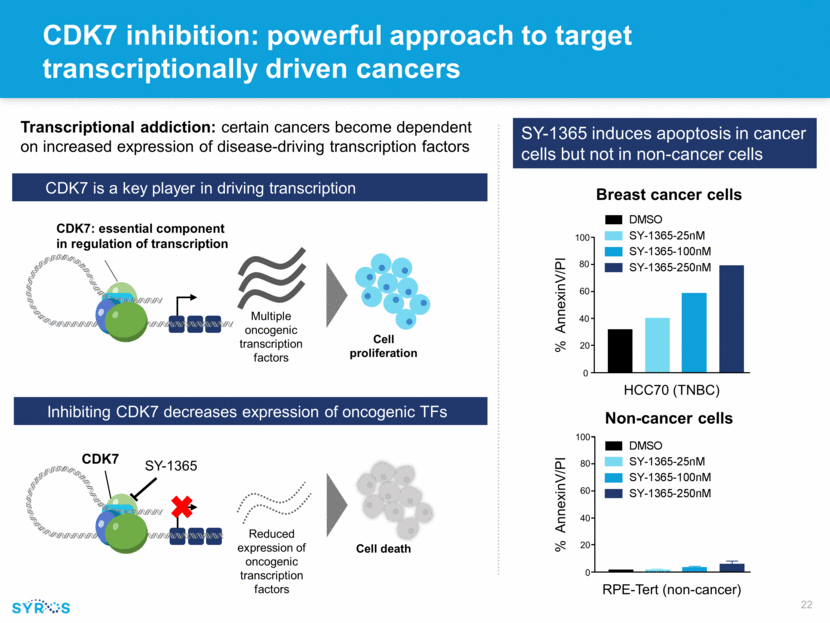
Reduced expression of oncogenic transcription factors Cell death CDK7 inhibition: powerful approach to target transcriptionally driven cancers Transcriptional addiction: certain cancers become dependent on increased expression of disease-driving transcription factors CDK7 is a key player in driving transcription Inhibiting CDK7 decreases expression of oncogenic TFs Multiple oncogenic transcription factors Cell proliferation CDK7: essential component in regulation of transcription CDK7 SY-1365 RPE-Tert (non-cancer) 100 20 0 40 60 80 Non-cancer cells % AnnexinV/PI SY-1365 induces apoptosis in cancer cells but not in non-cancer cells Breast cancer cells HCC70 (TNBC) % AnnexinV/PI 100 20 0 40 60 80 RPE-Tert BJ-Tert 0 20 40 60 80 100 DMSO SY-1365-25nM SY-1365-100nM SY-1365-250nM RPE-Tert BJ-Tert 0 20 40 60 80 100 DMSO SY-1365-25nM SY-1365-100nM SY-1365-250nM
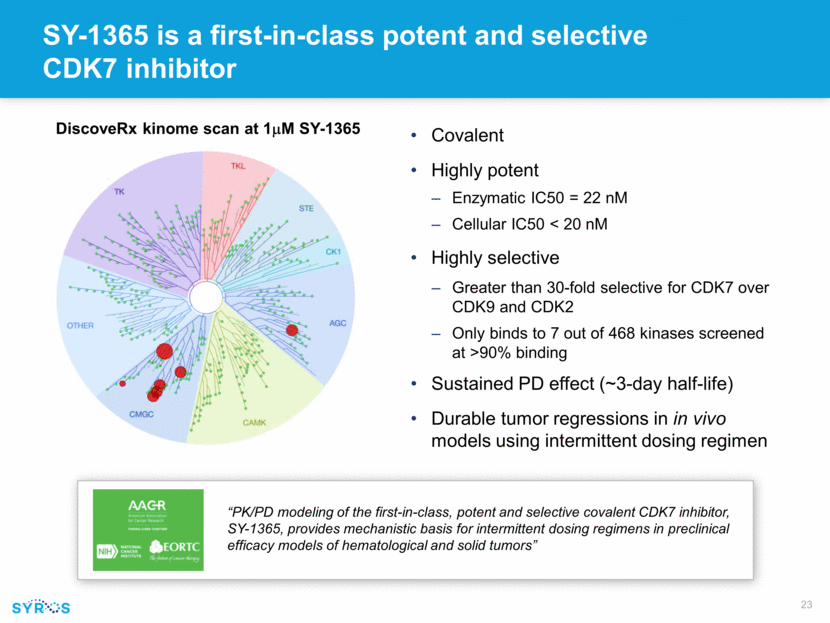
SY-1365 is a first-in-class potent and selective CDK7 inhibitor Covalent Highly potent Enzymatic IC50 = 22 nM Cellular IC50 < 20 nM Highly selective Greater than 30-fold selective for CDK7 over CDK9 and CDK2 Only binds to 7 out of 468 kinases screened at >90% binding Sustained PD effect (~3-day half-life) Durable tumor regressions in in vivo models using intermittent dosing regimen DiscoveRx kinome scan at 1mM SY-1365 “PK/PD modeling of the first-in-class, potent and selective covalent CDK7 inhibitor, SY-1365, provides mechanistic basis for intermittent dosing regimens in preclinical efficacy models of hematological and solid tumors”
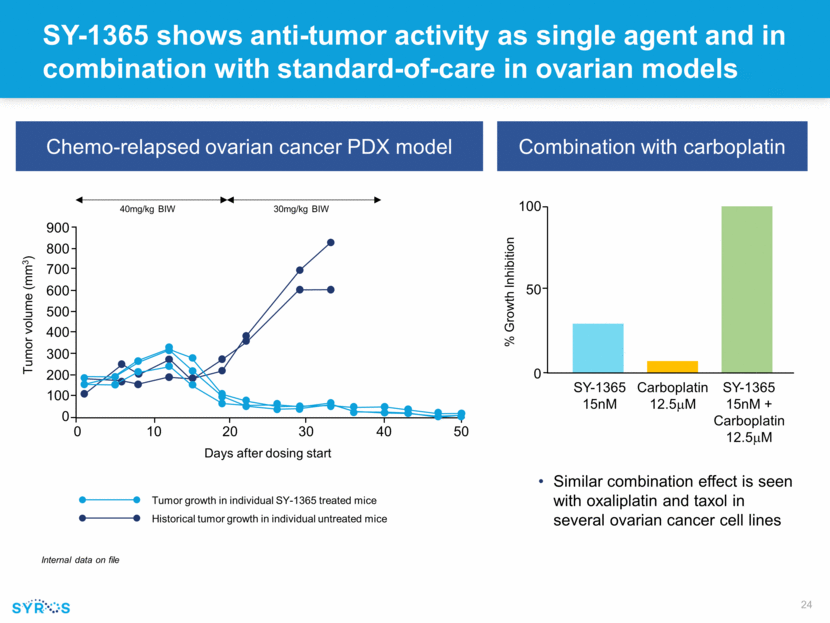
SY-1365 shows anti-tumor activity as single agent and in combination with standard-of-care in ovarian models Chemo-relapsed ovarian cancer PDX model Internal data on file 900 800 700 600 500 400 300 200 100 0 0 10 20 30 40 50 Tumor volume (mm3) 40mg/kg BIW 30mg/kg BIW Days after dosing start Tumor growth in individual SY-1365 treated mice Historical tumor growth in individual untreated mice Combination with carboplatin Similar combination effect is seen with oxaliplatin and taxol in several ovarian cancer cell lines SY-1365 15nM Carboplatin 12.5mM SY-1365 15nM + Carboplatin 12.5mM 100 50 0 % Growth Inhibition
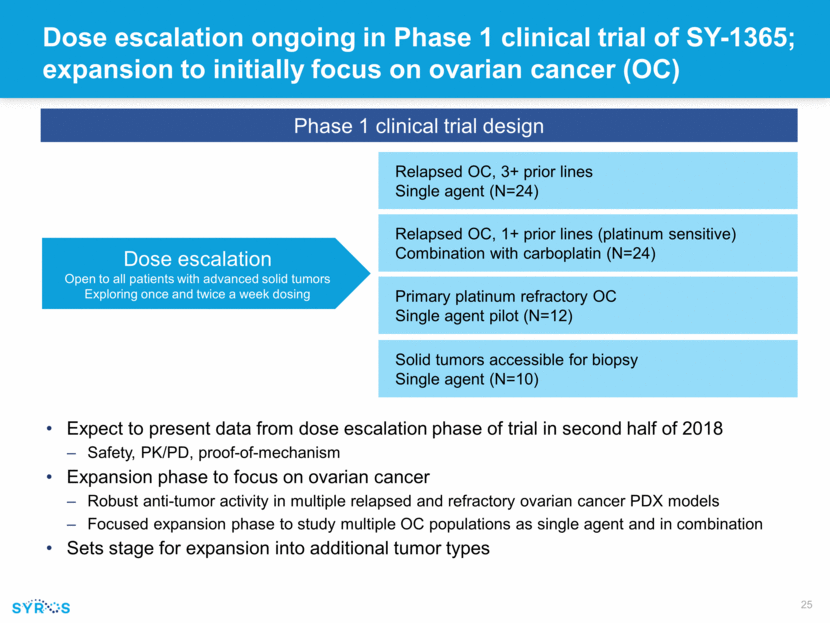
Dose escalation ongoing in Phase 1 clinical trial of SY-1365; expansion to initially focus on ovarian cancer (OC) Expect to present data from dose escalation phase of trial in second half of 2018 Safety, PK/PD, proof-of-mechanism Expansion phase to focus on ovarian cancer Robust anti-tumor activity in multiple relapsed and refractory ovarian cancer PDX models Focused expansion phase to study multiple OC populations as single agent and in combination Sets stage for expansion into additional tumor types Dose escalation Open to all patients with advanced solid tumors Exploring once and twice a week dosing Phase 1 clinical trial design Relapsed OC, 3+ prior lines Single agent (N=24) Relapsed OC, 1+ prior lines (platinum sensitive) Combination with carboplatin (N=24) Primary platinum refractory OC Single agent pilot (N=12) Solid tumors accessible for biopsy Single agent (N=10)
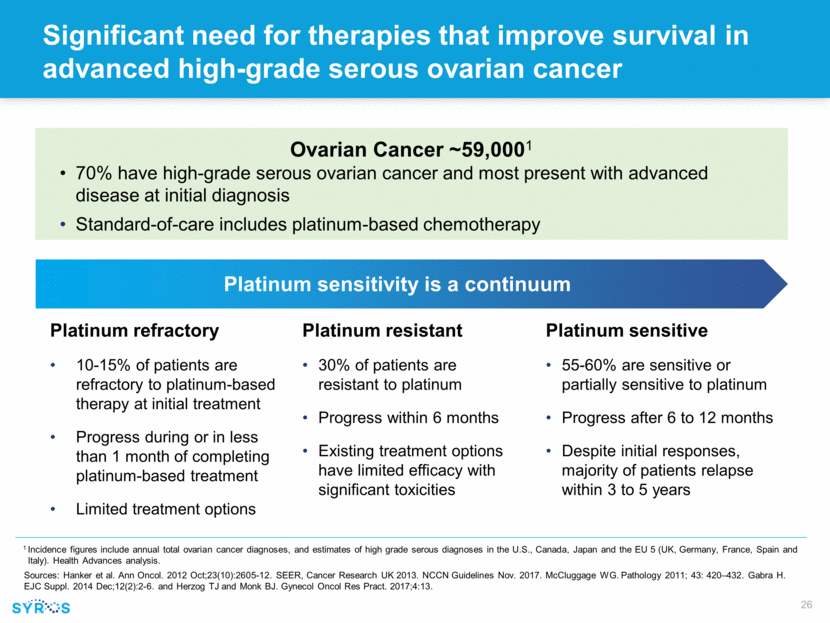
Significant need for therapies that improve survival in advanced high-grade serous ovarian cancer Platinum sensitive 55-60% are sensitive or partially sensitive to platinum Progress after 6 to 12 months Despite initial responses, majority of patients relapse within 3 to 5 years Platinum resistant 30% of patients are resistant to platinum Progress within 6 months Existing treatment options have limited efficacy with significant toxicities Platinum refractory 10-15% of patients are refractory to platinum-based therapy at initial treatment Progress during or in less than 1 month of completing platinum-based treatment Limited treatment options Ovarian Cancer ~59,0001 70% have high-grade serous ovarian cancer and most present with advanced disease at initial diagnosis Standard-of-care includes platinum-based chemotherapy 1 Incidence figures include annual total ovarian cancer diagnoses, and estimates of high grade serous diagnoses in the U.S., Canada, Japan and the EU 5 (UK, Germany, France, Spain and Italy). Health Advances analysis. Sources: Hanker et al. Ann Oncol. 2012 Oct;23(10):2605-12. SEER, Cancer Research UK 2013. NCCN Guidelines Nov. 2017. McCluggage WG. Pathology 2011; 43: 420–432. Gabra H. EJC Suppl. 2014 Dec;12(2):2-6. and Herzog TJ and Monk BJ. Gynecol Oncol Res Pract. 2017;4:13. Platinum sensitivity is a continuum
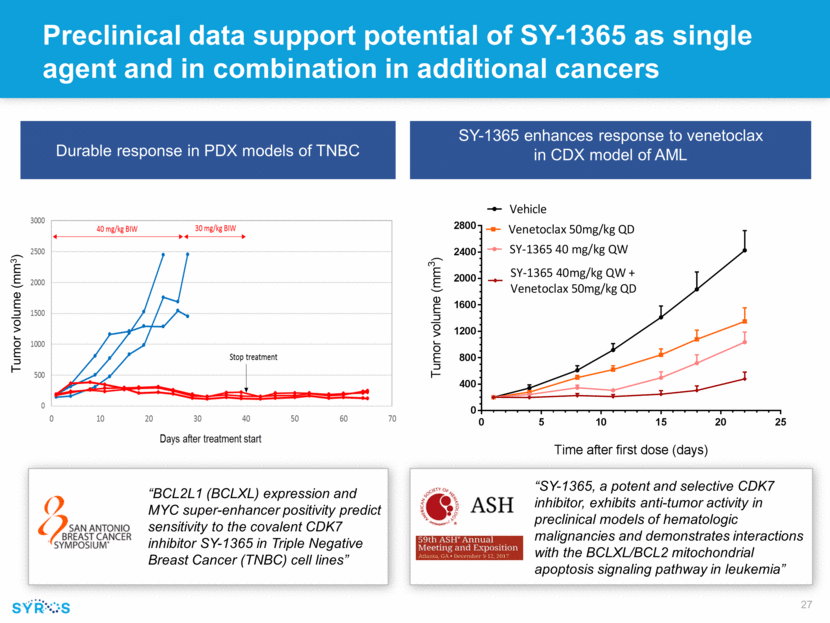
Preclinical data support potential of SY-1365 as single agent and in combination in additional cancers SY-1365 enhances response to venetoclax in CDX model of AML “BCL2L1 (BCLXL) expression and MYC super-enhancer positivity predict sensitivity to the covalent CDK7 inhibitor SY-1365 in Triple Negative Breast Cancer (TNBC) cell lines” “SY-1365, a potent and selective CDK7 inhibitor, exhibits anti-tumor activity in preclinical models of hematologic malignancies and demonstrates interactions with the BCLXL/BCL2 mitochondrial apoptosis signaling pathway in leukemia” Tumor volume (mm3) Durable response in PDX models of TNBC 0 5 10 15 20 25 0 400 800 1200 1600 2000 2400 2800 Vehicle Venetoclax 50mg/kg QD SY-1365 40 mg/kg QW SY-1365 40mg/kg QW + Venetoclax 50mg/kg QD Time after first dose (days) T u m o r v o l u m e ( m m 3 )
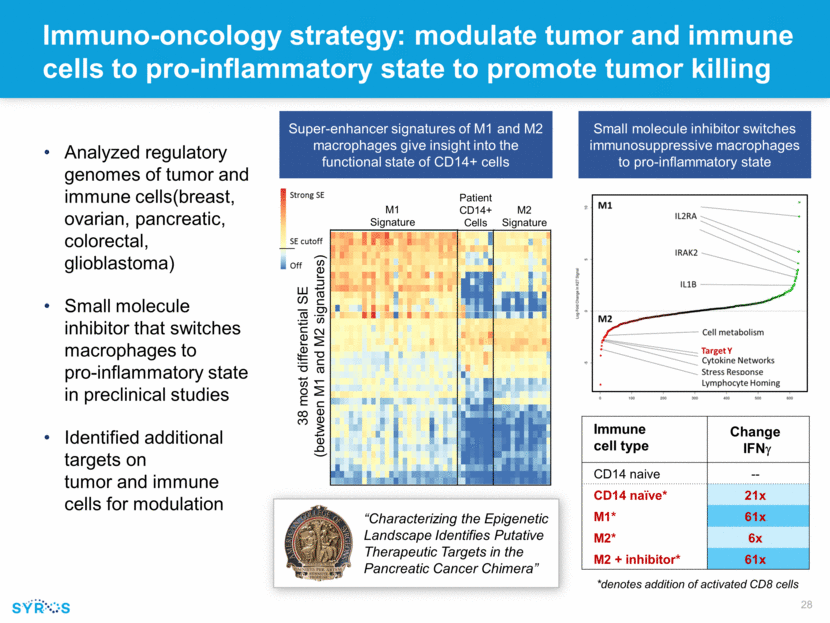
Immuno-oncology strategy: modulate tumor and immune cells to pro-inflammatory state to promote tumor killing Immune cell type Change IFNg CD14 naive -- CD14 naïve* 21x M1* 61x M2* 6x M2 + inhibitor* 61x *denotes addition of activated CD8 cells Analyzed regulatory genomes of tumor and immune cells(breast, ovarian, pancreatic, colorectal, glioblastoma) Small molecule inhibitor that switches macrophages to pro-inflammatory state in preclinical studies Identified additional targets on tumor and immune cells for modulation “Characterizing the Epigenetic Landscape Identifies Putative Therapeutic Targets in the Pancreatic Cancer Chimera” M1 Signature M2 Signature Patient CD14+ Cells Small molecule inhibitor switches immunosuppressive macrophages to pro-inflammatory state Super-enhancer signatures of M1 and M2 macrophages give insight into the functional state of CD14+ cells 38 most differential SE (between M1 and M2 signatures)
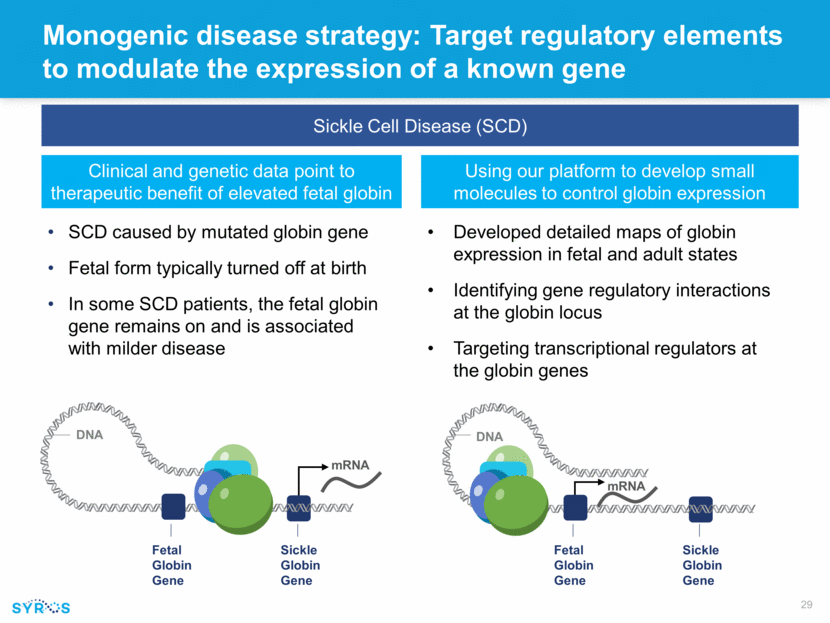
mRNA Monogenic disease strategy: Target regulatory elements to modulate the expression of a known gene Sickle Cell Disease (SCD) Clinical and genetic data point to therapeutic benefit of elevated fetal globin Using our platform to develop small molecules to control globin expression Sickle Globin Gene DNA mRNA Fetal Globin Gene Sickle Globin Gene DNA Fetal Globin Gene SCD caused by mutated globin gene Fetal form typically turned off at birth In some SCD patients, the fetal globin gene remains on and is associated with milder disease Developed detailed maps of globin expression in fetal and adult states Identifying gene regulatory interactions at the globin locus Targeting transcriptional regulators at the globin genes
Company Building

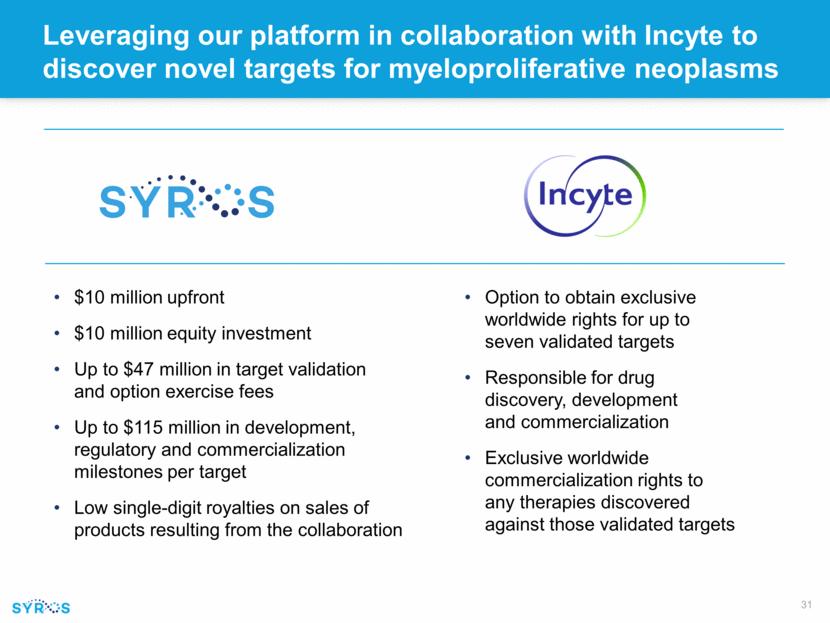
Leveraging our platform in collaboration with Incyte to discover novel targets for myeloproliferative neoplasms Option to obtain exclusive worldwide rights for up to seven validated targets Responsible for drug discovery, development and commercialization Exclusive worldwide commercialization rights to any therapies discovered against those validated targets $10 million upfront $10 million equity investment Up to $47 million in target validation and option exercise fees Up to $115 million in development, regulatory and commercialization milestones per target Low single-digit royalties on sales of products resulting from the collaboration
Strategic Collaborations In-licensing Pursuing strategic collaborations to maximize potential of our gene control platform Programs Continue to drive SY-1425 and SY-1365 to key value inflection points Assess partnerships post value inflections Platform Leverage gene control platform for target and drug discovery collaborations outside our focus areas Leverage platform for collaborations within our focus areas where partner’s expertise allows us to accelerate or expand our efforts Identify novel genomically defined subsets for patient selection and stratification Continue to be opportunistic on drugs that may have activity in our proprietary differential enhancer profiling
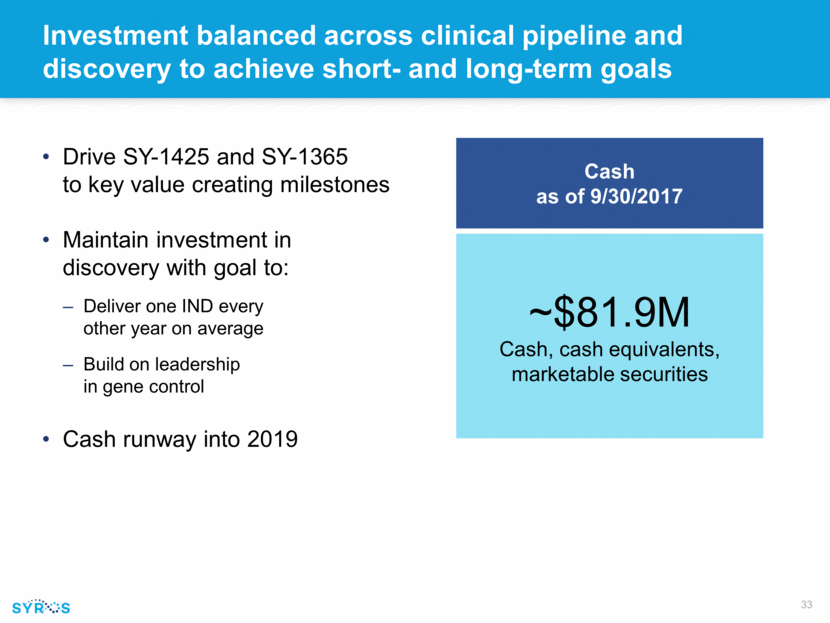
Investment balanced across clinical pipeline and discovery to achieve short- and long-term goals Drive SY-1425 and SY-1365 to key value creating milestones Maintain investment in discovery with goal to: Deliver one IND every other year on average Build on leadership in gene control Cash runway into 2019 ~$81.9M Cash, cash equivalents, marketable securities Cash as of 9/30/2017
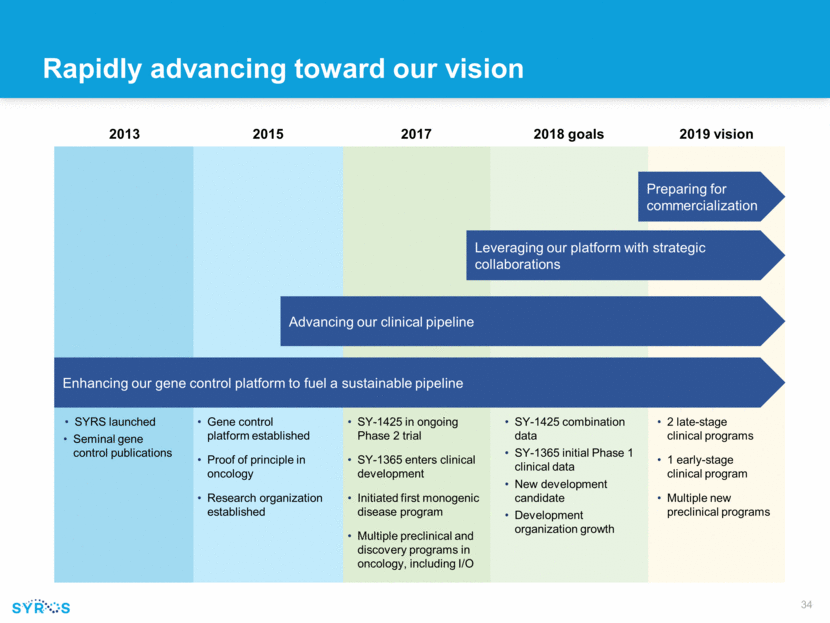
Rapidly advancing toward our vision SYRS launched Seminal gene control publications Gene control platform established Proof of principle in oncology Research organization established 2015 SY-1425 in ongoing Phase 2 trial SY-1365 enters clinical development Initiated first monogenic disease program Multiple preclinical and discovery programs in oncology, including I/O 2 late-stage clinical programs 1 early-stage clinical program Multiple new preclinical programs 2019 vision 2013 2017 Advancing our clinical pipeline Enhancing our gene control platform to fuel a sustainable pipeline Preparing for commercialization Leveraging our platform with strategic collaborations SY-1425 combination data SY-1365 initial Phase 1 clinical data New development candidate Development organization growth 2018 goals
[LOGO]
